Research Report
Regulatory Mechanisms of Graft Longevity: Insights from Genetically Modified Pig Organs 
 Author
Author  Correspondence author
Correspondence author
Journal of Vaccine Research, 2024, Vol. 14, No. 3
Received: 21 Apr., 2024 Accepted: 25 May, 2024 Published: 06 Jun., 2024
This study focuses on exploring the regulatory mechanisms that enhance graft longevity through genetically modified pig organs. The discussion centers on genetic modifications designed to improve immune compatibility and graft survival. Key gene editing technologies, such as CRISPR/Cas9, have enabled precise modifications of the pig genome, reducing immunogenicity and improving physiological compatibility. Experimental studies have shown the effectiveness of these modifications in preventing organ rejection and enhancing organ function in non-human primates and preliminary human trials. This study also addresses ethical considerations, including animal welfare and long-term genetic stability, as well as the regulatory frameworks governing xenotransplantation. It emphasizes the importance of interdisciplinary research, the development of immune tolerance strategies, and the integration of bioengineering approaches. This study highlights the potential of genetically modified pig organs as a viable solution to the organ shortage crisis, paving the way for future clinical applications.
1 Introduction
Organ transplantation has become an effective treatment for patients with end-stage organ failure. However, the shortage of donor organs results in many patients losing their lives while waiting for a suitable match. To address this challenge, scientists have explored xenotransplantation, specifically using animal organs to replace human ones, with pigs being a focal point due to the similarity of their organs to humans. Despite the challenges of immune rejection, gene-editing technologies like CRISPR/Cas9 have shown potential in modifying pig genetics to reduce immunogenicity and increase survival rates (Lei et al., 2022).
The success of xenotransplantation hinges on overcoming immune rejection. While traditional immunosuppressive therapies offer some relief, they come with significant side effects. Researchers have managed to knock out the GGTA1 gene in pigs, effectively reducing the expression of α-Gal antigens and lowering the risk of hyperacute rejection. Additionally, the introduction of human complement regulatory proteins such as CD55 and CD59 has significantly inhibited the activation of the complement system, reducing acute rejection (Cooper et al., 2019).
Beyond immune rejection, this research also focuses on the long-term survival and functional maintenance of transplants. Genetic modifications have enhanced the transplant's resistance to ischemia-reperfusion injury and used immunoregulatory genes to mitigate chronic inflammatory responses. Scientists are also studying how to balance the functionality of the transplant with the immunosuppressive treatment needs of patients, ensuring the stability and safety of genetic modifications (Sykes and Sachs, 2019).
This study reviews the main research progress and challenges in transgenic pig organ transplantation, exploring the molecular mechanisms that regulate the lifespan of transplants. It aims to provide a theoretical basis and reference for future xenotransplantation research and clinical applications. By deeply analyzing the roles of genetic modification and immune regulation, this study not only advances the scientific field but also offers new approaches and technological paths to address the global organ shortage. Additionally, the discussion on ethical and regulatory issues lays the groundwork for the safety and efficacy of this emerging technology.
2 Background on Genetically Modified Pig Organs
2.1 Rationale for using pigs in xenotransplantation
Pigs are considered ideal donors for xenotransplantation due to their anatomical and physiological similarities to humans. The size and function of pig organs are comparable to those of human organs, making them suitable for transplantation purposes. Additionally, pigs have a relatively short gestation period and produce large litters, which ensures a sustainable and scalable source of donor organs (Lei et al., 2022). This characteristic is crucial in addressing the severe shortage of human donor organs.
Moreover, pigs can be raised in controlled environments, minimizing the risk of zoonotic infections that could potentially complicate transplantation outcomes. This controlled breeding allows for better health monitoring and management of the donor animals, ensuring that they are free from specific pathogens that could be harmful to human recipients (Pan et al., 2019). The similarity in blood pressure, heart rate, and organ function between pigs and humans further supports the use of pigs in xenotransplantation.
The ethical considerations also favor pigs over non-human primates. While non-human primates are biologically closer to humans, their use raises significant ethical concerns. Pigs, on the other hand, are already extensively used in agriculture and medical research, making their use in xenotransplantation more ethically acceptable and less controversial (Cooper et al., 2019).
2.2 Overview of genetic modifications for improving organ compatibility
Genetic modifications are essential for overcoming the immunological barriers that typically cause rejection of pig organs when transplanted into humans. One of the primary targets for genetic modification is the α 1,3-galactosyltransferase (GGTA1) gene, which is responsible for producing the Gal antigen. This antigen is a major cause of hyperacute rejection in xenotransplantation. By knocking out the GGTA1 gene, the expression of the Gal antigen is eliminated, significantly reducing the risk of hyperacute rejection (Petersen et al., 2016).
In addition to knocking out problematic genes, genetic engineering has introduced human genes into pigs to enhance the compatibility of pig organs with the human immune system. For instance, the expression of human complement regulatory proteins such as CD46, CD55, and CD59 helps protect the transplanted organ from immune attack by inhibiting the complement cascade, which is part of the immune response that leads to organ rejection (Lei et al., 2022).
Further modifications include the introduction of anti-inflammatory and anti-coagulation genes to address other immune and physiological barriers. The expression of human thrombomodulin and endothelial cell protein C receptor in pigs helps prevent coagulation issues that can arise after transplantation, improving the overall success rates of xenotransplantation (Fischer et al., 2016).
2.3 Current status and advancements in pig organ transplantation research
The field of pig organ xenotransplantation has seen significant advancements in recent years, with genetically modified pigs showing promising results in preclinical studies. For example, triple-gene modified pigs, which lack major xenoantigens and express multiple human regulatory proteins, have demonstrated improved survival rates and function in non-human primate models. These pigs are bred to express human complement and coagulation regulatory proteins, reducing both hyperacute and acute vascular rejection (Cooper et al., 2019).
Recent research has also focused on refining genetic modifications to address remaining challenges such as chronic rejection and long-term graft function. For instance, new techniques like CRISPR/Cas9 are being used to make precise and efficient genetic edits, further improving the compatibility and function of pig organs for transplantation into humans (Kararoudi et al., 2018).
Moreover, the first clinical trials involving pig-to-human xenotransplantation are on the horizon, with studies showing that kidneys from genetically modified pigs can function effectively in brain-dead human recipients for extended periods without signs of hyperacute rejection (Montgomery et al., 2022). These advancements represent a significant step forward in making xenotransplantation a viable solution to the organ shortage crisis.
3 Mechanisms of Graft Longevity
3.1 Definition and importance of graft longevity
Graft longevity refers to the duration a transplanted organ remains functional and free from significant rejection or failure in the recipient's body. It is a critical measure of the success of organ transplantation, directly correlating with improved patient outcomes and quality of life. Achieving long-term graft survival reduces the need for re-transplantations, which in turn lowers healthcare costs and alleviates the strain on the limited supply of donor organs (Sykes and Sachs, 2019).
The importance of graft longevity is underscored by the challenges of chronic rejection and graft loss, which remain significant despite advancements in immunosuppressive therapies. Chronic rejection involves a complex interplay of immunological and non-immunological factors, leading to gradual deterioration and eventual failure of the graft. Enhancing graft longevity is therefore a primary goal in transplantation research, aiming to improve long-term patient survival and overall health outcomes (Rosales and Colvin, 2019).
Furthermore, graft longevity impacts not only the individual patient but also the broader healthcare system. Long-term graft survival reduces the frequency of hospital visits and the need for continuous medical interventions, thus contributing to better resource allocation and patient care management (Pan et al., 2019).
3.2 Biological and immunological factors influencing graft longevity
Several biological and immunological factors are crucial in determining the longevity of a graft. Key biological factors include the health and viability of the donor organ, ischemia-reperfusion injury, and the presence of pre-existing conditions in the recipient. Ischemia-reperfusion injury occurs when the blood supply returns to the tissue after a period of ischemia, causing oxidative stress and inflammation that can damage the graft. Strategies to mitigate this injury are critical for enhancing graft survival (Cooper et al., 2019).
Immunological factors are paramount in graft longevity, with the recipient's immune system playing a central role in graft rejection. The three primary immune responses include hyperacute rejection, acute cellular rejection, and chronic rejection. Hyperacute rejection occurs within minutes to hours post-transplantation, mediated by pre-existing antibodies against the donor antigens. Acute cellular rejection involves T-cell mediated immune responses leading to graft inflammation and damage, typically within the first few months after transplantation. Chronic rejection is a slow, progressive process involving both cellular and humoral immune responses, ultimately leading to long-term graft failure (Lei et al., 2022).
Additionally, the role of inflammation and immune regulation is significant in graft survival. Persistent inflammation can lead to tissue damage and fibrosis, reducing graft functionality over time. Effective management of immune responses and inflammation is therefore essential for prolonging graft longevity (Ekser et al., 2015).
3.3 Role of genetic modifications in enhancing graft survival
Genetic modifications in donor pigs have shown substantial promise in enhancing graft survival and longevity. These modifications aim to address immunological barriers and improve the compatibility of pig organs with the human immune system. One of the most impactful genetic modifications is the knockout of the GGTA1 gene, which eliminates the expression of the α-Gal antigen, a major target of pre-existing human antibodies. This modification significantly reduces the risk of hyperacute rejection, thereby enhancing graft survival (Petersen et al., 2016).
In addition to eliminating problematic antigens, the introduction of human complement regulatory proteins such as CD46, CD55, and CD59 into the pig genome has been successful in mitigating complement-mediated damage to the graft. These proteins help regulate the complement system, an integral part of the immune response, thus preventing excessive immune attacks on the transplanted organ (Fischer et al., 2016).
Further advancements include the insertion of genes that enhance the anti-inflammatory and anti-apoptotic properties of the graft. For example, the expression of human heme oxygenase-1 (HO-1) in genetically modified pigs provides cytoprotective effects, reducing ischemia-reperfusion injury and improving the overall resilience of the transplanted organ. These genetic modifications collectively contribute to longer graft survival and improved transplant outcomes (Coe et al., 2020).
4 Key Genetic Modifications in Pig Organs
4.1 Immunomodulatory genes (e.g., GGTA1, CMAH, β4GalNT2)
In xenotransplantation, immune rejection is a major barrier. To address this issue, scientists have edited multiple immunomodulatory genes. The GGTA1 gene encodes α1,3-galactosyltransferase (α-Gal), a major xenoantigen. Human immune systems recognize and attack cells expressing α-Gal, leading to hyperacute rejection. Knocking out the GGTA1 gene can eliminate α-Gal expression, significantly reducing the risk of hyperacute rejection.
The CMAH gene encodes N-glycolylneuraminic acid hydroxylase, producing the Neu5Gc antigen, a key factor in immune response. Knocking out CMAH can reduce human natural antibody binding, thus lowering the potential for immune rejection. Similarly, the β4GalNT2 gene encodes β-1,4-N-acetylgalactosaminyltransferase, producing the Sda antigen, which also plays a role in immune reactions. Eliminating these genes significantly improves graft survival and compatibility(Cooper et al., 2019).
Li et al. (2021) successfully used CRISPR/Cas9 technology to knock out GGTA1, CMAH, and β4GalNT2 in pig endothelial cells. These modified cells showed lower antigenicity in vitro, demonstrating the potential of multigene editing in xenotransplantation (Li et al., 2021). By targeting these key immunomodulatory genes, the immune response against pig organs in humans is significantly reduced, making xenotransplantation a more viable and effective solution.
4.2 Anti-inflammatory and anti-apoptotic genes
To enhance graft tolerance to the immune system, various anti-inflammatory and anti-apoptotic genes have been introduced. These genes not only reduce immune attacks on the graft but also improve its post-transplant survival. The human heme oxygenase-1 (HO-1) gene, for example, enhances graft survival through its antioxidant and anti-inflammatory properties. Ahrens et al. (2015) integrated HO-1 into GGTA1 knockout pigs, and these transgenic kidneys exhibited significant resistance to rejection and ischemia-reperfusion injury during ex vivo perfusion with human blood.
Anti-apoptotic genes such as A20 and Bcl-2 have also been incorporated into pig genomes to inhibit apoptosis and extend graft survival. The A20 gene encodes a protein with anti-inflammatory effects, regulating the NF-κB signaling pathway to reduce inflammation in the graft. Cooper et al. (2019) introduced A20 and other anti-inflammatory genes into transgenic pigs, resulting in significantly improved graft survival in non-human primates.
Heat shock protein 70 (HSP70) has also been investigated to enhance graft survival. Hryhorowicz et al. (2017) found that HSP70 expression reduced cellular stress responses and protected cells, demonstrating significant benefits for graft survival. Incorporating anti-inflammatory and anti-apoptotic genes can substantially improve graft survival and functionality, providing robust protection against immune attacks and harsh transplant environments (Hryhorowicz et al., 2017).
4.3 Genes promoting resistance to ischemia-reperfusion injury
Ischemia-reperfusion injury (IRI) is a common complication post-transplantation, severely affecting graft function and survival. To mitigate this damage, several genes have been introduced to enhance resistance to IRI. The heme oxygenase-1 (HO-1) gene, for instance, has proven effective in reducing oxidative stress and inflammation. Fischer et al. (2016) used gene editing to integrate HO-1 into multi-gene modified pigs, resulting in organs with improved resistance to IRI post-transplant.
The A20 gene plays a crucial role in reducing apoptosis and inflammation. By regulating the NF-κB signaling pathway, A20 reduces inflammatory responses, protecting grafts from IRI. Cooper et al. (2019) introduced A20 along with other anti-inflammatory genes into transgenic pigs, leading to improved graft survival in non-human primates.
Heat shock protein 70 (HSP70) also protects cells from IRI by stabilizing cellular proteins and preventing aggregation and apoptosis. Coe et al. (2020) demonstrated that pig livers modified to express HSP70 showed significant resistance to IRI during ex vivo perfusion with human blood, highlighting the potential of this gene in xenotransplantation.
5 Regulatory Pathways Involved in Graft Longevity
5.1 Immune response regulation and graft rejection mechanisms
In xenotransplantation, immune responses, including both innate and adaptive immunity, play crucial roles in graft rejection. Figure 1 illustrates the key immune participants in these rejection processes and outlines potential strategies to improve graft outcomes. The interaction between these immune components involves a multifaceted approach combining genetic modifications in donor pigs and targeted immunosuppressive treatments in recipients. By addressing both innate and adaptive immune responses, these strategies aim to enhance graft longevity and improve outcomes in xenotransplantation.
|
Figure 1 Key immunological players involved in xenograft rejection and possible strategies to improve graft outcome (Adapted from Vadori and Cozzi, 2015) Image caption: Figure 1 clearly illustrates the key players in both the innate and adaptive immune systems involved in xenograft rejection, along with various strategies to improve graft outcomes through genetic modifications and immunosuppressive approaches. The combination of these strategies helps to reduce rejection, extend graft survival, and enhance the success rates of xenotransplantation. (Adapted from Vadori and Cozzi, 2015) |
One key element of innate immunity is the complement system, which can trigger hyperacute rejection responses. To mitigate such responses, genetically engineered pigs typically lack specific carbohydrate xenoantigens (such as GGTA1, CMAH, and β4GalNT2) known to elicit strong human immune reactions. Additionally, transgenic pig organs express human complement regulatory proteins (such as CD46 and CD55) to help control complement activity. Inhibitors of the C3 complement component, like Cp40, are used to reduce tissue damage caused by the activation of neutrophils. Natural Killer (NK) cells and macrophages also participate in immune responses by recognizing and attacking xenogeneic cells, and transgenic pigs expressing human immune modulatory proteins (such as HLA-E, HLA-G, β2-microglobulin, and CD47) effectively reduce these cells' attacks (Rosales and Colvin, 2019).
In adaptive immunity, T cells and B cells are critical for graft rejection. Co-stimulatory blockade therapies, such as the CD40-CD40L pathway blockade, inhibit the activation and proliferation of T cells, thereby extending graft survival. Genetically modified pigs reduce the immunogenicity of cells, lowering the antibody response from B cells. Furthermore, the use of soluble polymers like Gas914 and specific immunosuppressants (such as anti-CD19, anti-CD20, bortezomib), as well as modulators of B cell activating factors (such as BAFF/APRIL inhibitors), are effective strategies for controlling B cell activity (Singh et al., 2018).
The combined application of these strategies, including genetic modification and immunosuppressive treatment, is key to improving graft outcomes and extending their lifespan. Through these methods, it is possible to significantly reduce the rejection responses in xenotransplantation, enhancing its success rate.
5.2 Inflammatory pathways and their modulation
Inflammation is a significant factor in graft rejection and failure. The modulation of inflammatory pathways is essential for prolonging graft survival. Genetically modified pigs often express anti-inflammatory genes such as human heme oxygenase-1 (HO-1) and A20, which help mitigate the inflammatory responses induced by the graft. HO-1 has been shown to have protective effects against oxidative stress and inflammation, thereby enhancing graft survival (Fischer et al., 2016).
In addition, regulatory macrophages (Mregs) play a critical role in controlling inflammation. Mregs secrete anti-inflammatory cytokines like interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), which help suppress immune responses and promote the development of regulatory T cells. This approach has been shown to reduce the required dosages and durations of immunosuppressive medications, potentially improving graft outcomes (Hoang and Kim, 2023).
Further advancements in the modulation of inflammatory pathways involve the use of gene editing technologies to remove or add specific inflammatory mediators. For instance, modifying pigs to express human anti-inflammatory proteins such as thrombomodulin can help control thrombotic and inflammatory reactions, which are common in xenotransplantation. These modifications have been shown to improve graft function and survival in non-human primate models (Singh et al., 2018).
5.3 Cellular stress response and protection mechanisms
Cellular stress responses play a pivotal role in graft survival, particularly in response to ischemia-reperfusion injury, which occurs during the transplantation process. Genetic modifications that enhance cellular protection mechanisms can significantly improve graft outcomes. For example, the expression of heat shock proteins (HSPs) such as HSP70 helps stabilize cellular proteins and prevent apoptosis under stress conditions, thus enhancing graft viability (Coe et al., 2020).
Additionally, anti-apoptotic genes like Bcl-2 and A20 have been introduced into pig organs to prevent programmed cell death triggered by transplantation-related stress. These genes help maintain cellular integrity and function, thereby extending graft survival. Studies have shown that organs from pigs expressing these genes exhibit lower levels of apoptosis and improved overall function in transplanted models (Hryhorowicz et al., 2017).
Another strategy involves the use of antioxidants to combat oxidative stress. Genes that enhance the production of antioxidants can help neutralize reactive oxygen species (ROS) generated during ischemia-reperfusion, thereby protecting the graft from oxidative damage. This approach has shown promise in experimental models, leading to better graft function and longer survival times (Fischer et al., 2016).
6 Insights from Experimental Studies
6.1 Case studies on genetically modified pig organ transplants
Experimental studies on genetically modified pig organs provide key insights into overcoming challenges in xenotransplantation. Notably, one important study showcased successful kidney transplants from pigs genetically engineered to knock out the alpha-1,3-galactosyltransferase (GGTA1) gene, crucial for preventing hyperacute rejection. This modification demonstrated potential in alleviating immediate rejection responses.
Biopsy samples from these transplanted kidneys were analyzed, with Figure 2 showing samples taken 54 hours after reperfusion. The results supported the initial success of these xenografts. Hematoxylin and eosin staining revealed normal-looking glomeruli and tubulointerstitium, with no signs of microvascular inflammation or lymphocytic infiltration, indicating effective integration and the absence of immediate immune rejection. Additionally, immunofluorescence microscopy of most samples showed minimal to no C4d staining in the peritubular capillaries, suggesting no antibody-mediated rejection, although one recipient displayed focal C4d staining, indicating some level of complement activation. Ultrastructural imaging further confirmed the structural integrity of the transplanted kidneys, displaying well-preserved glomerular basement membranes and podocyte foot processes. These findings confirm that the structural aspects of the kidneys were maintained post-transplantation, contributing to overall graft function (Montgomery et al., 2022).
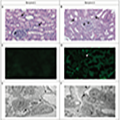 Figure 2 Photomicrographs of Biopsy Samples from Pig Kidneys Explanted from the Recipients 54 Hours after Reperfusion (Adapted from Montgomery et al., 2022) |
These case studies highlight the potential of genetically modified pig organs in human recipients, emphasizing the effectiveness of specific genetic modifications like the GGTA1 knockout in reducing rejection risks. The generally positive short-term results, along with occasional immune challenges such as focal C4d staining, underline the need for continued refinement of immunosuppressive strategies. These findings lay a foundation for future research aimed at enhancing graft longevity and functionality in xenotransplantation through combined genetic and immunosuppressive interventions(Singh et al., 2018).
6.2 Experimental results on graft survival and function
Genetic modification of pigs can significantly enhance the survival and function of transplants. For example, human complement regulatory proteins (such as CD46 and CD55) and coagulation regulatory proteins (such as thrombomodulin) expressed in genetically modified pigs are crucial in preventing acute rejection and coagulation disorders. These modifications enable pig kidneys to function for a long time in non-human primates, with some grafts surviving for over a year (Cooper et al., 2019).
Further research by Coe et al. (2020) involved ex vivo perfusion of genetically modified pig livers with human blood. The livers demonstrated prolonged function and improved biochemical parameters compared to non-modified pig livers. The genetically modified livers showed reduced antibody and complement deposition, highlighting the effectiveness of specific genetic modifications in improving graft compatibility and function (Coe et al., 2020).
A study on pig-to-human liver xenotransplantation using genetically modified pigs with multiple gene knockouts (GGTA1, CMAH, and β4GalNT2) showed improved survival and function of the grafts. The modifications helped mitigate hyperacute rejection and inflammation, with the grafts exhibiting near-normal liver function during the perfusion period.
6.3 Comparative analysis of different genetic modifications and their outcomes
Comparative analyses of different genetic modifications have provided valuable insights into the most effective strategies for enhancing graft survival and function. For instance, pigs with triple knockouts of GGTA1, CMAH, and β4GalNT2 combined with the expression of human complement regulatory proteins (CD46, CD55) and coagulation regulatory proteins (thrombomodulin) have shown superior outcomes in terms of graft survival and function compared to pigs with fewer modifications. These multiple modifications address various immune and coagulation challenges simultaneously, leading to more stable and functional grafts (Kemter et al., 2020).
Another comparative study highlighted the importance of anti-inflammatory and anti-apoptotic genes in improving graft outcomes. Pigs expressing human heme oxygenase-1 (HO-1) and A20 genes exhibited reduced inflammatory responses and lower levels of apoptosis in transplanted organs, resulting in better graft survival and function compared to those without these modifications (Fischer et al., 2016).
The role of macrophage inhibitory proteins, such as human CD47, has also been emphasized in comparative studies. Pigs expressing human CD47 showed reduced phagocytosis by recipient macrophages, leading to enhanced graft tolerance and prolonged survival in non-human primate models. These findings suggest that combining multiple genetic modifications targeting different pathways can synergistically improve the outcomes of xenotransplantation.
7 Ethical and Regulatory Considerations
7.1 Ethical issues in the use of genetically modified animals for transplantation
The use of genetically modified animals, particularly pigs, for organ transplantation raises several ethical concerns. One primary issue is animal welfare. Genetic modifications often involve procedures that can cause pain and suffering to animals, such as invasive surgeries and prolonged isolation. For instance, modifications aimed at reducing immune rejection may require multiple genetic alterations and rigorous testing, which can impact the animals' quality of life. Critics argue that these practices may violate the principles of humane treatment and animal integrity (Eriksson et al., 2018).
Another ethical issue pertains to the concept of naturalness. The introduction of human genes into animal genomes is seen by some as crossing a moral boundary, altering the essence of the species. This raises questions about the intrinsic value of animals and whether such modifications are a form of exploitation. There is also the debate about whether the benefits to humans justify the ethical costs to animals, especially when alternative solutions, such as improving human organ donation systems, exist (Johnson, 2022).
Moreover, ethical dilemmas arise from the potential long-term ecological impacts of genetically modified organisms (GMOs). The release of GMOs into the environment, even in controlled settings, poses risks of unintended gene transfer and ecological disruptions. These concerns necessitate stringent ethical oversight and continuous monitoring to mitigate potential harms.
7.2 regulatory frameworks governing xenotransplantation
Regulatory frameworks for xenotransplantation are essential to ensure the safety and ethical integrity of using genetically modified organs. In the European Union, the precautionary principle guides the regulation of GMOs, emphasizing the need to avoid potential risks when scientific understanding is incomplete. This principle is applied to both environmental and health risks associated with xenotransplantation (Anyshchenko, 2019).
In the United States, the Food and Drug Administration (FDA) regulates genetically modified animals used for medical purposes under the New Animal Drug provisions. The FDA's guidelines require extensive testing for safety, efficacy, and ethical considerations before approval. This includes assessing the potential for zoonotic disease transmission, immune response, and long-term health effects on recipients (Polcz and Lewis, 2018).
China has also been advancing its regulatory framework, focusing on boosting the commercialization of genetically modified animals while ensuring safety and public acceptance. Regulatory reforms have been aimed at streamlining approval processes while maintaining stringent safety standards to foster innovation and public trust (Fan et al., 2021).
7.3 Public perception and societal implications
Public perception plays a crucial role in the acceptance and implementation of xenotransplantation. Many people express concerns about the ethical implications of using genetically modified animals, including fears of unnaturalness and potential health risks. Addressing these concerns requires transparent communication from scientists and policymakers, emphasizing the benefits and addressing the ethical safeguards in place (Cengiz and Wareham, 2019).
Education and engagement with the public are vital for fostering trust. This includes explaining the scientific rationale behind genetic modifications, the rigorous testing protocols, and the ethical frameworks governing these practices. Public forums, debates, and educational campaigns can help demystify the technology and address misconceptions (Garas et al., 2015).
Societal implications also extend to issues of equity and access. There is a concern that advanced medical technologies like xenotransplantation could exacerbate existing health disparities if access is limited to affluent populations. Ensuring fair distribution and addressing affordability will be critical in realizing the full potential of this technology to benefit all segments of society (Hodge, 2018).
8 Challenges and Limitations
8.1 Technical challenges in genetic modification and transplantation
Genetic modification of pigs for xenotransplantation involves intricate technical processes that pose significant challenges. One primary technical challenge is achieving precise and stable integration of human genes into the pig genome. Techniques like CRISPR/Cas9, although highly effective, can still result in unintended genomic modifications. For example, the insertion of human genes to prevent hyperacute rejection or enhance compatibility must be accurately targeted to avoid disrupting essential pig genes or causing off-target effects. A study on ex vivo gene editing of kidneys highlighted the difficulty in silencing major histocompatibility complex (MHC) antigens without triggering adverse immune responses during organ perfusion (Yuzefovych et al., 2020).
Another challenge is ensuring the viability and function of the genetically modified organs. Genetic modifications often involve multiple gene edits, each carrying the risk of unintended consequences that could impair organ function. The production of transgenic pigs with multiple modifications requires sophisticated breeding and screening processes to ensure the desired traits are reliably expressed without compromising overall health and viability (Mohiuddin et al., 2016).
Additionally, the transplantation process itself poses technical hurdles. The surgical techniques for xenotransplantation are complex and require meticulous planning and execution. Ensuring proper vascular anastomosis and minimizing ischemia-reperfusion injury are critical for the success of the transplant. Innovations in surgical techniques and pre-transplant conditioning protocols are necessary to enhance graft survival and function (Soltys et al., 2017).
8.2 Potential off-target effects and genetic stability
One of the significant concerns with genetic modification is the potential for off-target effects, which can lead to unintended mutations and genetic instability. Off-target effects occur when the gene-editing tool, such as CRISPR/Cas9, introduces changes at unintended genomic sites. These off-target mutations can have deleterious effects, including the activation of oncogenes or the disruption of essential regulatory genes. A study on cytosine base editors revealed that these tools could induce numerous off-target single-nucleotide variants, highlighting the need for more precise gene-editing techniques (Zuo et al., 2019).
Ensuring the long-term genetic stability of modified organs is another critical challenge. Genetic modifications must remain stable across multiple generations of cells and throughout the lifespan of the transplanted organ. Instability in the inserted genes can lead to loss of function or the re-emergence of immunogenic epitopes, which can compromise graft survival. Techniques like DISCOVER-Seq are being developed to monitor and detect off-target effects in vivo, providing a more comprehensive understanding of the genomic stability of edited cells (Wienert et al., 2018).
Moreover, the genetic environment of the host can influence the stability and expression of the modified genes. Interactions between the host genome and the introduced genes can lead to unpredictable outcomes, necessitating thorough preclinical testing and long-term monitoring to ensure the safety and efficacy of the genetically modified organs (Jin et al., 2019).
8.3 Long-term monitoring and management of graft function
Long-term monitoring and management of graft function are crucial for the success of xenotransplantation. Continuous assessment is necessary to detect early signs of rejection, infection, or other complications that could compromise the graft. Advances in biomarkers and non-invasive monitoring techniques are essential for real-time evaluation of graft health. For instance, a three-gene assay has been developed to monitor immune quiescence and predict graft stability in kidney transplant patients, highlighting the potential of gene expression profiling in graft monitoring (Roedder et al., 2015).
The management of immunosuppression is another critical aspect. Balancing the need to prevent rejection while minimizing the side effects of long-term immunosuppressive therapy is challenging. Over-immunosuppression can lead to increased susceptibility to infections and malignancies, whereas under-immunosuppression can result in graft rejection. Personalized immunosuppressive regimens based on individual patient profiles and the use of novel immunomodulatory therapies are being explored to improve long-term outcomes (Martínez-Llordella and Lechler, 2015).
Furthermore, the development of operational tolerance, where the recipient's immune system accepts the graft without the need for continuous immunosuppression, is a long-term goal. Achieving and maintaining this state requires sophisticated immunomonitoring tools and strategies to ensure that the graft remains accepted by the host immune system over time (Azad et al., 2018).
9 Future Directions and Perspectives
9.1 Emerging trends and innovations in genetic modification for xenotransplantation
The field of xenotransplantation is rapidly evolving with numerous emerging trends and innovations in genetic modification. One significant trend is the use of CRISPR/Cas9 technology, which has revolutionized the ability to create precise and multiple genetic modifications in pigs. This technology enables the deletion of key antigens that cause hyperacute rejection, such as GGTA1, CMAH, and β4GalNT2, as well as the addition of human complement and coagulation regulatory genes like CD46, CD55, and thrombomodulin. These advancements have significantly improved graft survival and reduced rejection rates (Fischer et al., 2016).
Another innovation is the development of pigs with a broader array of genetic modifications to address not just immune compatibility but also physiological and metabolic compatibility. For example, recent efforts have focused on engineering pigs to express human cytokines and growth factors, which can promote better integration and function of the transplanted organs in human recipients (Lei et al., 2022). Additionally, gene-editing strategies are being used to eliminate porcine endogenous retroviruses (PERVs), which pose a risk of cross-species viral transmission. These efforts are crucial for making xenotransplantation safer and more acceptable for clinical use (Cowan and Tector, 2017).
The combination of advanced genetic editing techniques and improved understanding of xenograft rejection mechanisms is paving the way for more reliable and longer-lasting xenotransplantation outcomes. Researchers are continuously exploring new genetic targets and refining editing methods to enhance the efficacy and safety of xenotransplants (Kararoudi et al., 2018).
9.2 Potential breakthroughs in enhancing graft longevity
Potential breakthroughs in enhancing graft longevity are on the horizon, driven by innovative research and technological advancements. One promising area is the induction of immune tolerance, where the recipient's immune system is conditioned to accept the xenograft without long-term immunosuppression. Techniques such as mixed chimerism, where donor and recipient hematopoietic cells coexist, and thymic transplantation are being explored to promote tolerance and reduce the need for immunosuppressive drugs (Llore et al., 2018).
Another breakthrough is the use of bioengineering and regenerative medicine approaches to enhance graft longevity. Researchers are developing bioengineered scaffolds and organs that can be seeded with genetically modified pig cells, creating hybrid organs that are more compatible with human physiology. These bioengineered organs can potentially overcome many of the current limitations associated with traditional xenotransplantation (Eissa et al., 2022).
Furthermore, advancements in immunosuppressive therapies and the development of novel biologics targeting specific pathways involved in graft rejection are likely to improve outcomes. These therapies, combined with genetic modifications, can synergistically enhance graft survival and function. The use of biomarkers for early detection of rejection and real-time monitoring of graft health is also expected to play a crucial role in managing xenotransplants more effectively.
9.3 Interdisciplinary research and collaboration opportunities
The future of xenotransplantation relies heavily on interdisciplinary research and collaboration. Collaborative efforts between geneticists, immunologists, bioengineers, and clinicians are essential to address the multifaceted challenges of xenotransplantation. For instance, integrating knowledge from immunology and genetics can lead to the development of more targeted and effective genetic modifications (Cozzi et al., 2021).
International collaboration is also crucial for standardizing protocols and regulatory frameworks, which will facilitate the transition from preclinical to clinical studies. Sharing data and resources across institutions can accelerate the pace of innovation and ensure that breakthroughs in one area are quickly translated into clinical practice (Ekser et al., 2017).
Additionally, partnerships with biotechnology and pharmaceutical companies can drive the commercialization and scalability of xenotransplantation technologies. These collaborations can help in the development of new immunosuppressive drugs, gene-editing tools, and bioengineered organs, making xenotransplantation more accessible and practical for widespread clinical use (Mohiuddin et al., 2019). By fostering a collaborative and interdisciplinary approach, the xenotransplantation field can overcome current limitations and achieve significant advancements, ultimately providing a viable solution to the organ shortage crisis.
10 Concluding Remarks
The use of genetically modified pig organs for xenotransplantation has shown significant promise in addressing the shortage of human organs available for transplantation. Advances in genetic modification techniques, such as CRISPR/Cas9, have enabled the precise and efficient editing of pig genomes to eliminate immunogenic antigens and introduce human regulatory proteins, thereby improving graft survival and reducing rejection. Experimental studies have demonstrated the potential for these genetically modified organs to function effectively in non-human primates and, in some cases, even humans. Moreover, the introduction of multiple genetic modifications has been crucial in addressing various immunological and physiological barriers, leading to prolonged graft longevity and functionality.
The progress in xenotransplantation suggests that clinical trials involving genetically modified pig organs are becoming increasingly viable. Future research should focus on refining genetic modification techniques to further enhance graft compatibility and reduce off-target effects. Additionally, studies on immune tolerance induction and the development of novel immunosuppressive therapies are essential to improve long-term graft survival. The integration of bioengineering approaches to create hybrid organs and the use of advanced monitoring tools for early detection of graft rejection will be crucial in advancing the field. As these technologies evolve, the potential for routine clinical use of xenotransplants will grow, offering a sustainable solution to the organ shortage crisis.
Continued research is imperative to address the remaining challenges in xenotransplantation, including technical, immunological, and ethical issues. Interdisciplinary collaboration and international partnerships will be key in accelerating progress and ensuring the successful translation of preclinical findings to clinical practice. Ethical considerations must remain at the forefront of this research, with a focus on animal welfare, genetic integrity, and societal implications. Transparent communication with the public and stakeholders is essential to build trust and acceptance of xenotransplantation. By prioritizing both scientific innovation and ethical responsibility, the field can move closer to realizing the full potential of genetically modified pig organs in saving human lives.
Acknowledgement
Sincerely thank the peer studyers for their valuable feedback and suggestions on my research.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Anyshchenko A., 2019, The precautionary principle in EU regulation of GMOs: socio-economic considerations and ethical implications of biotechnology, Journal of Agricultural and Environmental Ethics, 32: 855-872.
https://doi.org/10.1007/s10806-019-09802-2
Azad T., Donato M., Heylen L., Liu A., Shen-Orr S., Sweeney T., Maltzman J., Naesens M., and Khatri P., 2018, Inflammatory macrophage-associated 3-gene signature predicts subclinical allograft injury and graft survival, JCI Insight, 3(2): e95659.
https://doi.org/10.1172/jci.insight.95659
PMid:29367465 PMCid:PMC5821209
Cengiz N., and Wareham C., 2019, Pig-to-human xenotransplantation: overcoming ethical obstacles, South African Journal of Bioethics and Law, 12: 66-71.
https://doi.org/10.7196/sajbl.2019.v12i2.00677
Cooper D., Hara H., Iwase H., Yamamoto T., Li Q., Ezzelarab M., Federzoni E., Dandro A., and Ayares D., 2019, Justification of specific genetic modifications in pigs for clinical organ xenotransplantation, Xenotransplantation, 26(4): e12516.
https://doi.org/10.1111/xen.12516
PMid:30989742 PMCid:PMC10154075
Coe T., Detelich D., Rickert C., Carroll C., Serifis N., Matheson R., Raigani S., Rosales I., Qin W., Kan Y., Layer J., Youd M., Westlin W., Kimura S., Azimzadeh A., Yang L., and Markmann J., 2020, Prolonged survival of genetically modified pig livers during machine perfusion with human blood, Transplantation, 104(S3): S37.
https://doi.org/10.1097/01.tp.0000698436.68163.75
Cowan P., Cowan P., and Tector A., 2017, The resurgence of xenotransplantation, American Journal of Transplantation, 17: 2531-2536.
https://doi.org/10.1111/ajt.14311
PMid:28397351
Cozzi E., Schneeberger S., Bellini M., Berglund E., Böhmig G., Fowler K., Hoogduijn M., Jochmans I., Marckmann G., Marson L., Neuberger J., Oberbauer R., Pierson R., Reichart B., Scobie L., White C., and Naesens M., 2021, Organ transplants of the future: planning for innovations including xenotransplantation, Transplant International, 34(11): 1993-2421.
https://doi.org/10.1111/tri.14031
Eriksson S., Jonas E., Rydhmer L., and Röcklinsberg H., 2018, Invited study: breeding and ethical perspectives on genetically modified and genome edited cattle, Journal of Dairy Science, 101(1): 1-17.
https://doi.org/10.3168/jds.2017-12962
PMid:29102147
Ekser B., Li P., and Cooper D., 2017, Xenotransplantation: past, present, and future, Current Opinion in Organ Transplantation, 22: 513-521.
https://doi.org/10.1097/MOT.0000000000000463
Eissa N., Badrkhan S., Mohamed M., Shaban J., Shahban R., and Dawoud M., 2022, Xenotransplantation: past, present, and future directions, Highlights in BioScience, 5: bs202205.
https://doi.org/10.36462/H.BioSci.202205
Fan Z., Mu Y., Sonstegard T., Zhai X., Li K., Hackett P., and Zhu Z., 2021, Social acceptance for commercialization of genetically modified food animals, National Science Study, 8(8): nwab067.
https://doi.org/10.1093/nsr/nwab067
PMid:34691713 PMCid:PMC8363318
Fischer K., Kraner-Scheiber S., Petersen B., Rieblinger B., Buermann A., Flisikowska T., Flisikowski K., Christan S., Edlinger M., Baars W., Kurome M., Zakhartchenko V., Kessler B., Plotzki E., Szczerbal I., Świtoński M., Denner J., Wolf E., Schwinzer R., Niemann H., Kind A., and Schnieke A., 2016, Efficient production of multi-modified pigs for xenotransplantation by 'combineering', gene stacking and gene editing, Scientific Reports, 6: 29081.
https://doi.org/10.1038/srep29081
PMid:27353424 PMCid:PMC4926246
Garas L., Murray J., and Maga E., 2015, Genetically engineered livestock: ethical use for food and medical models, Annual Study of Animal Biosciences, 3: 559-575.
https://doi.org/10.1146/annurev-animal-022114-110739
PMid:25387117
Hodge D., 2018, Xenotransplantation, trust, and trustworthiness: ethical issues for African Americans, Ethics Medicine and Public Health, 7: 59-67.
https://doi.org/10.1016/J.JEMEP.2018.10.003
Hryhorowicz M., Zeyland J., Słomski R., and Lipinski D., 2017, Genetically modified pigs as organ donors for xenotransplantation, Molecular Biotechnology, 59: 435-444.
https://doi.org/10.1007/s12033-017-0024-9
PMid:28698981 PMCid:PMC5617878
Hoang T., and Kim J., 2023, Regulatory macrophages in solid organ xenotransplantation, Korean Journal of Transplantation, 37(4): 229-240.
https://doi.org/10.4285/kjt.23.0055
PMid:38115165 PMCid:PMC10772277
Jin S., Zong Y., Gao Q., Zhu Z., Wang Y., Qin P., Liang C., Wang D., Qiu J., Zhang F., and Gao C., 2019, Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice, Science, 364: 292-295.
https://doi.org/10.1126/science.aaw7166
PMid:30819931
Johnson L., 2022, Existing ethical tensions in xenotransplantation, Cambridge Quarterly of Healthcare Ethics, 31: 355-367.
https://doi.org/10.1017/S0963180121001055
PMid:35659820
Kararoudi M., Hejazi S., Elmas E., Hellström M., Kararoudi M., Padma A., Lee D., and Dolatshad H., 2018, Clustered regularly interspaced short palindromic repeats/Cas9 gene editing technique in xenotransplantation, Frontiers in Immunology, 9: 1711.
https://doi.org/10.3389/fimmu.2018.01711
PMid:30233563 PMCid:PMC6134075
Kemter E., Schnieke A., Fischer K., Cowan P., and Wolf E., 2020, Xeno-organ donor pigs with multiple genetic modifications-the more the better, Current Opinion in Genetics and Development, 64: 60-65.
https://doi.org/10.1016/j.gde.2020.05.034
PMid:32619817
Lei T., Chen L., Wang K., Du S., Gonelle-Gispert C., Wang Y., and Buhler L., 2022, Genetic engineering of pigs for xenotransplantation to overcome immune rejection and physiological incompatibilities: the first clinical steps, Frontiers in Immunology, 13: 1031185.
https://doi.org/10.3389/fimmu.2022.1031185
PMid:36561750 PMCid:PMC9766364
Li P., Walsh J., Lopez K., Isidan A., Zhang W., Chen A., Goggins W., Higgins N., Liu J., Brutkiewicz R., Smith L., Hara H., Cooper D., and Ekser B., 2021, Genetic engineering of porcine endothelial cell lines for evaluation of human-to-pig xenoreactive immune responses, Scientific Reports, 11: 13131.
https://doi.org/10.1038/s41598-021-92543-y
PMid:34162938 PMCid:PMC8222275
Llore N., Bruestle K., and Griesemer A., 2018, Xenotransplantation tolerance: applications for recent advances in modified swine, Current Opinion in Organ Transplantation, 23: 642-648.
https://doi.org/10.1097/MOT.0000000000000585
PMid:30379724 PMCid:PMC7010353
Mohiuddin M., DiChiacchio L., Singh A., and Griffith B., 2019, Xenotransplantation: a step closer to clinical reality, Transplantation, 103(3): 453-454.
https://doi.org/10.1097/TP.0000000000002608
PMid:30801425
Mohiuddin M., Singh A., Corcoran P., Iii M., Clark T., Lewis B., Hoyt R., Eckhaus M., III R., Belli A., Wolf E., Klymiuk N., Phelps C., Reimann K., Ayares D., and Horvath K., 2016, Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft, Nature Communications, 7: 11138.
https://doi.org/10.1038/ncomms11138
PMid:27045379 PMCid:PMC4822024
Montgomery R., Stern J., Lonze B., Tatapudi V., Mangiola M., Wu M., Weldon E., Lawson N., Deterville C., Dieter R., Sullivan B., Boulton G., Parent B., Piper G., Sommer P., Cawthon S., Duggan E., Ayares D., Dandro A., Fazio-Kroll A., Kokkinaki M., Burdorf L., Lorber M., Boeke J., Pass H., Keating B., Griesemer A., Ali N., Mehta S., and Stewart Z., 2022, Results of two cases of pig-to-Human kidney xenotransplantation, The New England journal of medicine, 386(20): 1889-1898.
https://doi.org/10.1056/NEJMoa2120238
PMid:35584156
Martinez-Llordella M, and Lechler R., 2015, Tracking donor-reactive T cells: perspectives for the development of tolerance protocols, Transplantation, 99(12): 2436-2437.
https://doi.org/10.1097/TP.0000000000000999
PMid:26627670
Petersen B., Frenzel A., Lucas-Hahn A., Herrmann D., Hassel P., Klein S., Ziegler M., Hadeler K., and Niemann H., 2016, Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes, Xenotransplantation, 23: 338-346.
https://doi.org/10.1111/xen.12258
PMid:27610605
Pan D., Liu T., Lei T., Zhu H., Wang Y., and Deng S., 2019, Progress in multiple genetically modified minipigs for xenotransplantation in China, Xenotransplantation, 26(1): e12492.
https://doi.org/10.1111/xen.12492
PMid:30775816
Petersen B., Frenzel A., Lucas-Hahn A., Herrmann D., Hassel P., Klein S., Ziegler M., Hadeler K., and Niemann H., 2016, Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes, Xenotransplantation, 23: 338-346.
https://doi.org/10.1111/xen.12258
PMid:27610605
Polcz S., and Lewis A., 2018, A menagerie of moral hazards: regulating genetically modified animals, The Journal of Law Medicine and Ethics, 46: 180-184.
https://doi.org/10.1177/1073110518766031
Rosales I., and Colvin R., 2019, The pathology of solid organ xenotransplantation, Current Opinion in Organ Transplantation, 24(5): 535-542.
https://doi.org/10.1097/MOT.0000000000000681
PMid:31348015
Roedder S., Li L., Alonso M., Hsieh S., Vu M., Dai H., Sigdel T., Bostock I., Macedo C., Metes D., Zeevi A., Shapiro, R., Salvatierra O., Scandling J., Alberú J., Engleman E., and Sarwal M., 2015, A Three-gene assay for monitoring immune quiescence in kidney transplantation, Journal of the American Society of Nephrology, 26(8): 2042-2053.
https://doi.org/10.1681/ASN.2013111239
PMid:25429124 PMCid:PMC4520154
Soltys K., Setoyama K., Tafaleng E., Gutiérrez A., Fong J., Fukumitsu K., Nishikawa T., Nagaya M., Sada R., Haberman K., Gramignoli R., Dorko K., Tahan V., Dreyzin A., Baskin K., Crowley J., Quader M., Deutsch M., Ashokkumar C., Shneider B., Squires R., Ranganathan S., Reyes-Múgica M., Dobrowolski S., Mazariegos G., Elango R., Stolz D., Strom S., Vockley G., Roy-Chowdhury J., Cascalho M., Guha C., Sindhi R., Platt J., and Fox I., 2017, Host conditioning and rejection monitoring in hepatocyte transplantation in humans, Journal of Hepatology, 66(5): 987-1000.
https://doi.org/10.1016/j.jhep.2016.12.017
PMid:28027971 PMCid:PMC5395353
Singh A., DiChiacchio L., Chan J., Lewis B., Thomas M., Corcoran P., Ayares D., Horvath K., and Mohiuddin M., 2018, Expression of human thrombomodulin on GTKO, CD46 Donor Pigs and costimulation blockade by anti CD40 antibody is critical for extending cardiac xenograft survival in non-human primates, Transplantation, 102(7): S741.
https://doi.org/10.1097/01.tp.0000543732.72476.8d
Sykes M., and Sachs D., 2019, Transplanting organs from pigs to humans, Science Immunology, 4(41): eaau6298.
https://doi.org/10.1126/sciimmunol.aau6298
PMid:31676497 PMCid:PMC7293579
Vadori M., and Cozzi E., 2015, The immunological barriers to xenotransplantation, Tissue Antigens, 86: 239-253.
https://doi.org/10.1111/tan.12669
PMid:26381044
Wienert B., Wyman S., Richardson C., Yeh C., Akçakaya P., Porritt M., Morlock M., Vu J., Kazane K., Watry H., Judge L., Conklin B., Maresca M., and Corn J., 2018, Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq, Science, 364: 286-289.
https://doi.org/10.1126/science.aav9023
PMid:31000663 PMCid:PMC6589096
Yuzefovych Y., Valdivia E., Rong S., Hack F., Rother T., Schmitz J., Bräsen J., Wedekind D., Moers C., Wenzel N., Gueler F., Blasczyk R., and Figueiredo C., 2020, Genetic engineering of the kidney to permanently silence MHC transcripts during ex vivo organ perfusion, Frontiers in Immunology, 11: 265.
https://doi.org/10.3389/fimmu.2020.00265
PMid:32140158 PMCid:PMC7042208
Zuo E., Sun Y., Wei W., Yuan T., Ying W., Sun H., Yuan L., Steinmetz L., Li Y., and Yang H., 2019, Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos, Science, 364: 289-292.
https://doi.org/10.1126/science.aav9973
PMid:30819928 PMCid:PMC7301308
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yun Liu
Related articles
. Xenotransplantation
. Genetic Modification
. Graft Longevity
. Immune Compatibility
. CRISPR/Cas9
Tools
. Post a comment
